By Sofiane Boukhalfa, PhD, project architect; and Navneeta Kaul, PhD, researcher; both with PreScouter
The modern world runs on lithium-based batteries. Numerous chemistries and novel technologies are being developed to counter the limitations of lithium-ion batteries though, including the high cost, raw materials sourcing and overheating. Chicago-based research intelligence firm PreScouter recently released a report detailing 10 new battery technologies poised to disrupt the market over the next decade and usher in the next wave of high-performance batteries. Here’s a high-level look at the report findings, including a review of these battery technologies most valuable to solar-plus-storage.

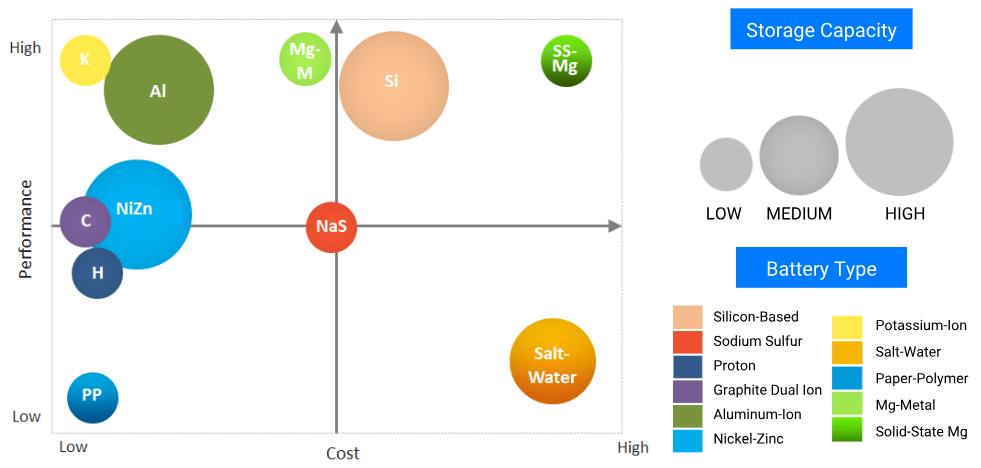
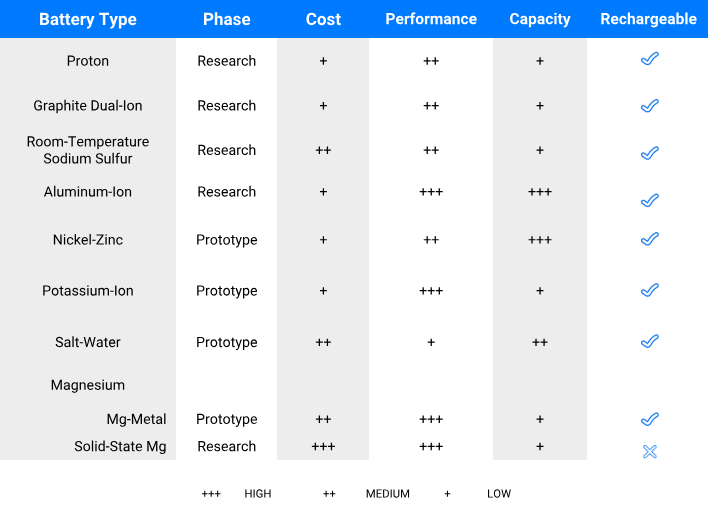
Ten battery technologies that could disrupt the solar-plus-storage market in the next five to 10 years. PreScouter
-
Silicon-based batteries
Li-ion batteries have traditionally used graphite anodes, but researchers and companies are now focusing on silicon anodes. The Si-dominant anodes can bind Li-ion 25-times more than the graphite ions. However, these batteries suffer from low electrical conductivity, a slow-diffusion rate and large volumetric fluctuations during lithiation. These limitations result in Si pulverization and instability of the solid electrolyte interphase (SEI).
Two primary strategies have been used to circumvent these challenges: nanotechnology and carbon coating. In the former method, various nano-sized Si anodes are used, which have a high surface area, improved cycle life and rate stability compared to bulk Si anodes. They can also withstand lithiation and delithiation without cracking. Carbon coating uses a combination of nanosized Si with different forms of carbon materials for generation of high-performance Si/C nanocomposite anodes. Recently, doped carbon with heteroatoms as coating agents have attracted a lot of interest. The heteroatom-doped Si-C electrodes bind Li ions more strongly than carbon atoms, leading to an excellent electrochemical performance with stable electrical conductivity.
Si-based batteries have generated a lot of commercial interest due to their potential for low costs and enhanced capabilities for cars and smartphones. The competition is fierce, with many startup companies, including Sila Nanotechnologies, Enovix, Angstron Materials and Enevate, to commercialize Si-dominant Li-Ion batteries.
-
Room-temperature sodium sulfur (RT-NaS) batteries
One of the most promising alternatives to lithium-sulfur batteries are sodium-sulfur batteries, due to similar physical and chemical properties of Na and Li ions. However, a high temperature (>300°C) is needed for battery operation. As a promising alternative, the low-cost RT-NaS battery system has generated extensive research interest for use in large-scale grid applications with enhanced safety. However, due to complex reactions within the battery, the RT-NaS batteries suffer from a lower theoretical capacity.
Various approaches have been used in 2018 to solve the problems of RT-NaS batteries.
- A team of researchers at MIT led by Dr. Sadoway focused on the membrane to solve the problem of the brittle and fragile nature of the beta alumina ceramic electrolyte membrane between the anode and cathode components of the RT-NaS. They demonstrated that a steel mesh coated with a solution of titanium nitride functions as stronger and more flexible material for industrial-scale storage systems. The approach opens up new avenues for battery design, as it can be applied to other molten-electrode battery chemistries as well.
- Researchers at the University of Wollongong, Australia, focused on the electrode design. They built an efficient sulfur cathode with atomic Cobalt anchored in the micropores of hollow carbon nanospheres. The synthesized cathode demonstrated excellent electrochemical performance.

Schematic illustration of the synthesis of the hollow carbon decorated with cobalt nanoparticles. Nature
- In recent research published in “Nature,” scientists used a multifunctional carbonate electrolyte with high electrochemical performance and increased safety. This approach could be applied to a wide range of Na-based rechargeable battery systems for the advancement of low-cost and high-performance energy storage devices.

Schematic illustration of the electrolytes with conventional 1M NaTFSI in PC electrolyte and (right) 2MNaTFSI in PC:FEC with 10mM InI3 additive electrolyte. Nature
Though RT-NaS batteries are still in the early phase of development, companies like Ambri, a spin-out company from MIT led by Dr. Sadoway, is working to improve the battery design. The next generation of NaS-based energy storage technologies could soon become a reality with the ongoing research efforts and approaches discussed above.
-
Proton batteries
Many research efforts have been devoted to the generation of high-performance proton exchange membrane (PEM) fuel cells. However, the viability of PEM fuel cells has been a challenge due to their high cost, transportation and storage of hydrogen gas.
A team of researchers at RMIT University recently reported the technical feasibility of a proton battery for the first time. It consists of two parts: a carbon electrode to store hydrogen or protons from water and a reversible PEM fuel cell to generate electricity from the hydrogen. The battery design is innovative, as it uses activated carbon for the electrode, which is cheap, abundant and structurally stable for hydrogen storage and a small volume of liquid acid inside the porous material that conducts protons to and from the membrane of the reversible cell. With this battery, a voltage of 1.8 V is achievable.

The novel battery concept as proposed in 2014 by Professor Andrews from RMIT. Graphical abstract from Professor Andrews research paper
Though a tremendous step for efficient hydrogen-powered energy production, the commercialization of this technology is still a long way off. The team estimates the availability of the battery to be within five to 10 years. ABB Marine and Sintef Ocean are also testing a megawatt-scale propulsion plant to power commercial and passenger ships using hydrogen fuel cells. As these batteries do not require Li-ion at all, aside from using platinum as a catalyst, the remaining materials are inexpensive and abundant and therefore could be a leading contender to the current Li-ion batteries.
-
Graphite dual-ion batteries
Dual-ion batteries (DIBs) that use metals other than lithium have attracted a lot of interest in recent years for large-scale stationary storage of electricity. Research efforts are on to increase the energy density of the DIBs by increasing the ionic content of the electrolyte and the ability of the electrodes to store charge.
- Researchers demonstrated a new Li-free graphite dual-ion battery using a graphite cathode and a potassium anode, known as graphite dual-ion battery (GDIB). The findings were published in “Nature Communications.” The team identified Li-free electrode-electrolyte combinations for DIB to increase the energy density of the cell. They used a concentrated electrolyte solution that demonstrated energy efficiency at par with Li-ion batteries.
- Using aluminum salt electrolytes, a research team has developed graphite-graphite dual-ion batteries (GGDIB) for the first time. The battery is inexpensive, environment-friendly and shows a superior cycle and rate performance for future energy storage applications.
- In another promising approach to DIBs, researchers at the South China University of Technology have reported the development of a Zn/graphite dual-ion battery. Due to the many attractive features of the ionic electrolyte, including suppressing dendrite formation on the Zn surface, low volatility, noninflammability, and high thermal stability, high-performance and safe Zn/graphite-ion batteries for industrial applications could become a reality soon.
-
Aluminum-ion batteries
Abundant, inexpensive, readily available and cheap, aluminum is being investigated as a potential replacement for Li-ion batteries. Swiss researchers from ETH Zurich have come up with two new technologies that are a stepping stone to the commercialization of Al-based batteries.
- The first is a corrosion-resistant coating material, titanium nitride (TiN) ceramic, for use in these batteries. The excellent oxidative stability of TiN-coated materials enabled these batteries to attain a high energy density, high coulombic efficiency, and high cycling ability. Due to the excellent corrosion resistance of TiN current collectors, they could even be used as high-voltage cathode materials in Mg-, Na-, or Li-ion batteries.
- Another promising solution is the use polypyrenes as a high-performance cathode material for Al-ion batteries. These batteries typically use a graphite-based cathode, which gets distorted due to the chloroaluminate anions. Using a custom-made cell, the researchers tested polypyrene and its derivative poly(nitropyrene-co-pyrene) as cathode materials and found that it stores the same amount of energy as a graphite cathode. Moreover, polypyrenes offer numerous other possibilities for developing rechargeable Al-ion batteries, including low cost, high abundance, production scalability, and compositional and structural tunability.
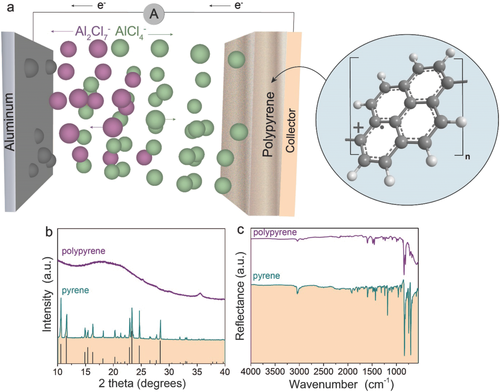
Schematic of the working principle of a rechargeable aluminum battery during charge with a polypyrene cathode and chloroaluminate ionic liquid. Advanced Materials
These research efforts show great promise towards commercializing Al-ion batteries for use as an inexpensive storage solution for the industry.
-
Nickel-zinc batteries
Nickel-zinc batteries are cost effective, safe, nontoxic, environment-friendly batteries that could compete with Li-ion batteries for energy storage. However, the main barrier for commercialization has been their low cycle life.
To address this problem, Chinese researchers from the Dalian University of Technology have developed a breakthrough in-situ cutting technique to improve the performance of Ni-Zn batteries by solving the issue of Zn electrode dissolution and suppressing the formation of dendrites. The team developed a novel graphene-ZnO hybrid electrode with the in-situ cutting technique, which can cut graphene directly into short nanoribbons. The strong interatomic interactions anchor Zn atoms onto graphene surfaces. This approach thoroughly fixes the issues of Zn electrode dissolution, dendrite formation, and performance.
With the ongoing research and approaches taken by companies, these batteries show immense potential for widespread commercial applications of electric vehicles (EVs) and energy storage.
-
Potassium-ion batteries
There have been a lot of recent breakthroughs to improve the electrochemical performance of potassium-ion batteries (KIBs). Three worth noting are listed below.
- A team of researchers from various institutions discovered a novel family of honeycomb-layered compounds with a general formula of K2M2TeO6 (where M=Ni, Mg, Co, etc. or a combination of at least two transition metals). These honeycomb structured potassium-based tellurate compounds are suitable for high-voltage cathode materials and are capable of inserting K ions into ionic liquids, making them excellent candidates for the development of high-energy KIBs.
- Similarly, another team at the University of Wollongong developed a high-performance KIB with a composite of few-layered antimony sulfide/carbon sheet (SBS/C) anode.
- Other promising approaches include focusing on the synergistic combination of the electrolyte and the electrode as well as developing suitable anode materials to design a high-performance KIB.
These novel approaches will help circumvent the limitations of the suitable host substrates for intercalating K ions and are a promising step towards attracting industrial investments for commercial applications.
-
Salt-water batteries
Water can conduct ions and be used to form rechargeable batteries. However, the chemical stability of water lasts up to 2.3 V, which is three times less than Lithium-ion batteries, limiting its use in EVs. These batteries could be suitable for stationary power-storage applications. To achieve the potential, researchers at the Swiss Materials Testing and Research Institute (Empa) used a specific salt called sodium bis(fluorosulfonyl)imide (FSI), which is very soluble in water. The salt-containing liquid has all the water molecules concentrated around the sodium cations in a hydrate shell, resulting in hardly any unbound water molecules present. This saline solution shows superior electrochemical stability of up to 2.6V, which is twice as high as other aqueous electrolytes. The prototype has shown promising results in the lab and can stand multiple charge-discharge cycles.
Similarly, researchers at Stanford have developed a low-cost, durable salt-water battery for solar- and wind-energy storage. These batteries are easy to develop, as they only need manganese sulfate, water, cheap industrial salt, and electrodes for the catalytic reactions. Moreover, the chemical reaction stores electrons as hydrogen gas for future use, illustrating its suitability for grid-scale applications. The performance of the prototype manganese-hydrogen battery could be scaled up and shows a solid performance of up to 10,000 cycles and an extended life span. The battery is in the process of getting patented by the researchers before commercial applications. It has generated a lot of industrial interest, and companies including Aquion Energy are working to make cheaper batteries for grid-level storage. BlueSky Energy uses Aquion’s salt-water technology for residential solar storage.
Although the current applications of the salt-water batteries are limited, they still offer several advantages, including safety, low cost and nontoxicity, for use in stationary storage systems.
-
Paper-polymer batteries
Paper-based microbial bio-batteries have generated widespread interest, as they are inexpensive, environment-friendly, and self-sustainable. They could have enormous applications in biosensors and future electronic devices. However, the main limitation is the low performance.
Recently, Seokheun Choi and a team of scientists developed a high-performance microbial battery engineered from a biodegradable paper-polymer substrate. The pores of the paper contained freeze-dried electric bacteria capable of exporting electrons as a by-product of respiration. To further improve the electric performance, the team incorporated a biodegradable polymer mixture into the paper. These hybrid paper-polymer microbial fuel cells show an enhanced power-to-cost ratio, with a shelf life of about four weeks without needing any additional conditioning or microorganisms. The technology is under patent application, and the team is seeking industrial investments for commercialization. Further improvements in design optimization could offer more versatility in the use of these batteries for numerous other applications.
-
Magnesium batteries
Mg-based batteries could compete with Li-ion in theory, due to a higher energy density capacity. However, Mg-based batteries are not rechargeable, as the reversible reaction requires a corrosive electrolyte that creates a barrier for Mg2+ ions.
For the first time, scientists at the Department of Energy’s National Renewable Energy Laboratory (NREL) presented a prototype of a rechargeable Mg-based battery. They generated an artificial Mg2+-conductive interface on the Mg anode surface. The interface protects the surface of the Mg anode while enabling the reversible cycling of an Mg/V2O5 fuel cell in a water-containing, carbonate-based electrolyte. The strategy significantly improves the battery performance of Mg-based batteries.
In another approach, a team of researchers at MIT, Berkeley and Argonne National Laboratory developed a solid-state material that conducts Mg ions faster, especially in the ternary spinel chalcogenide framework. This battery design requires further testing and research to enter the commercialization phase.
Top featured technologies for solar applications
Batteries used for solar applications require several characteristics beyond low cost. Capacity and power ratings for solar batteries will depend on energy and power density characteristics of the batteries. Additionally, metrics like the depth of discharge, overall lifetime, and efficiency of the battery will be crucial in determining which chemistries end up working for which specific niches/applications.
Though a lot of the batteries featured above are in the early phase of development, they could offer low-cost alternatives to Lithium-ion batteries for solar applications with a longer lifetime and a wide temperature range. Ni-Zn, Mg, Al-ion, NaS, graphite DIBs, KIB, proton and salt-water batteries could all play an important role. These are recyclable and are the subject of much research investigating how to optimize the chemistries with no undesirable side reactions. As such, they offer great promise for renewable-power storage. For example, BlueSky energy has already started using salt-water batteries for residential solar storage, with prices comparable to Lithium-ion batteries.
About the authors
Sofiane Boukhalfa, PhD, Project Architect, PresSouter
Sofiane leads the high-tech, aerospace and defense, and finance verticals at PreScouter. Sofiane earned his B.S. in Materials Science and Engineering from The University of Illinois at Urbana-Champaign, and his Ph.D. in Materials Science and Engineering from the Georgia Institute of Technology, where his research focused on nanotechnology and energy storage. Since graduating from Georgia Tech, he has worked as an emerging technology and business strategy consultant at several firms and for his own clients.
Navneeta Kaul, PhD, Researcher, PreScouter
Navneeta graduated with a PhD in biology from the University of Denver in August, 2018. The focus of her research was to understand the mechanism of local protein synthesis at the synapse which is important for memory formation in vertebrates. She has experience in using biochemical and molecular biology techniques like cloning, PCR, real-time PCR, western blotting, immunoprecipitation, live cell, and fixed cell imaging. She is passionate about communicating new technologies and research advances to a wider audience.






There is a company in the Netherlands that have made a biobased storage battery from sand, salt and tree leaves and is 100% recyclable and very cheap. Suwotec battery
The company that I’ve been trying to follow and kind of want to see a product that I can buy is NantEnergy. Zinc-air is perhaps old technology but because of that should be able to deliver cost-effective power now.
Still no predictive theories to how batteries actually work, so we must continue to just experiment.
So have have dozens and dozens of projects. No doubt hard to find and list all of them.
I believe batteries are almost a $68 billion business world wide. So many people are interested.
Vanadium redux batteries seem ideal for home storage and are scalable for grid application. These are already in use. They are stable, can be discharged and recharged indefinitely and the electrolyte is recyclable. Not usable for vehicles but perfect match with solar. Seems better than all 10 battery types discussed here. Why was it not on the list.
Vanadium Flow batteries don’t make the grade?
I think aluminum air batteries should have been included given their high energy density but otherwise a nice overview of the state of the art.
I am curious as to why XNRGI’s Lithium Metal battery is not included in this.
This contribution is about non-lithium batteries.
where does this one fit in?
https://spectrum.ieee.org/energywise/energy/renewables/does-new-glass-battery-accelerate-the-end-of-oil
Good info.